Prostate Cancer Lineage Plasticity is Driven by Epigenetic Alterations
by Shaghayegh Nouruzi
The progression of castration-resistant prostate cancer (CRPC) to neuroendocrine prostate cancer (NEPC) is accompanied by epigenetic plasticity, chromatin remodeling, histone modifications, and changing DNA methylation patterns. Key transcription factors, such as ASCL1, and chromatin modifiers such as EZH2 and DNMT, emerge as essential players in driving this aggressive stage of the disease.
Epigenetic Plasticity in castration-resistant prostate cancer (CRPC) to neuroendocrine prostate cancer (NEPC) Transition
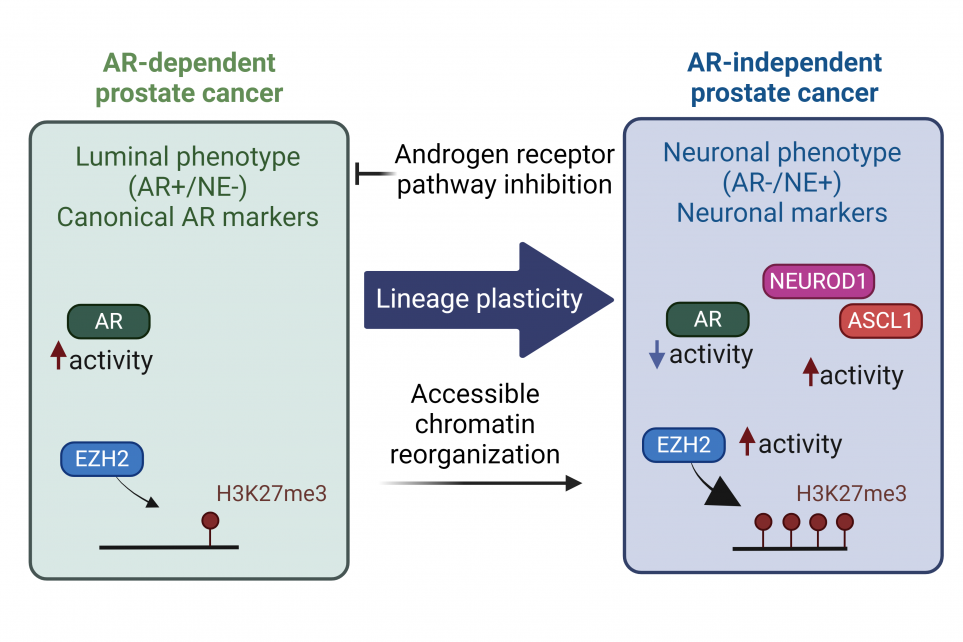
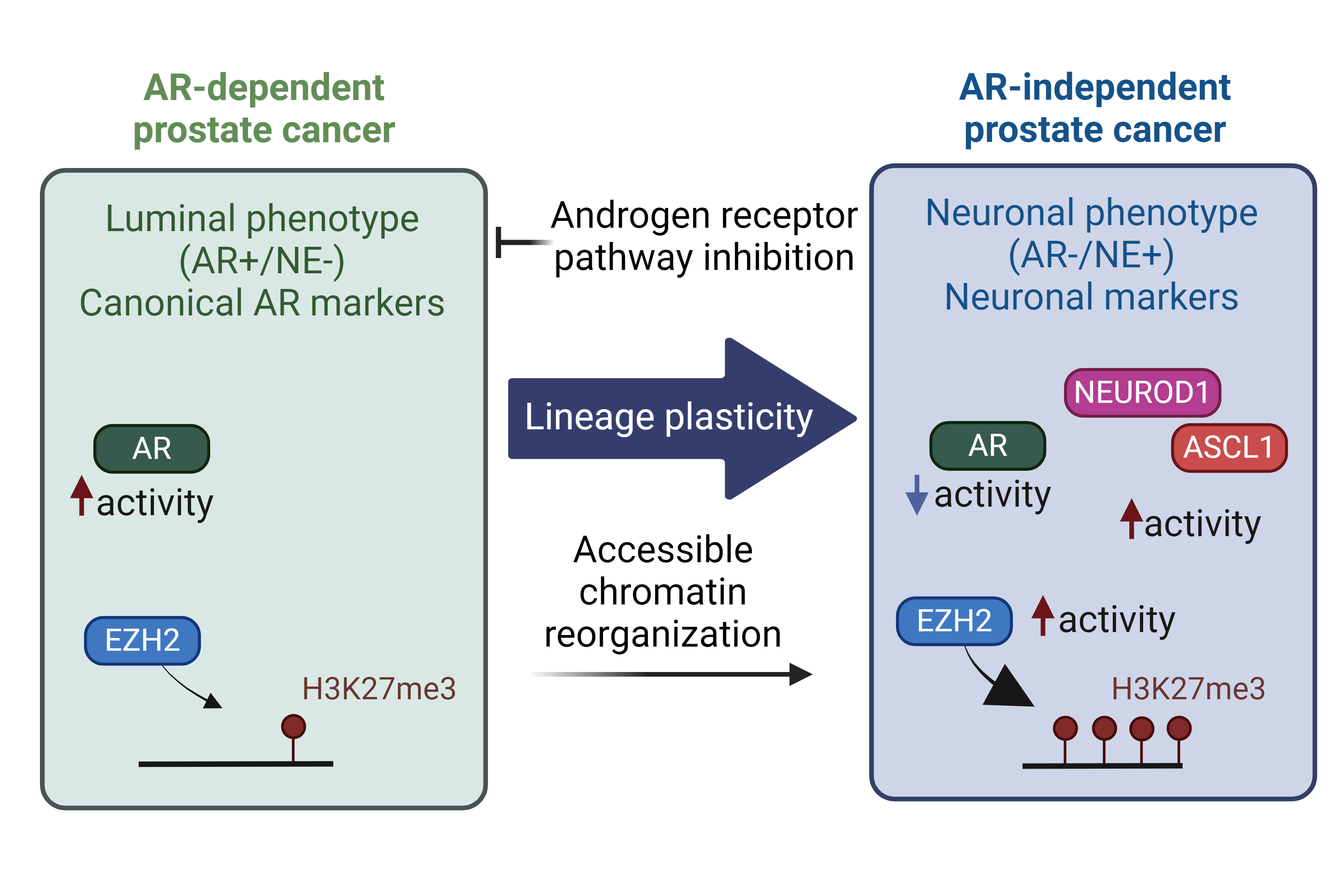
Despite the significant success of second-generation androgen receptor (AR) pathway inhibitors (ARPIs) (such as enzalutamide1 and abiraterone2), castration-resistant prostate cancers (CRPC) progress under prolonged androgen deprivation3. A subset of CRPC tumors evade the pressure of targeted therapies by shedding their dependence on AR (AR-independent)4. These new clones harbor distinct molecular profiles and unlock a new phenotype associated with stem-cell-like and neuronal characteristics5-10. Lineage plasticity underlies this transition to an alternative phenotype. This process is initially driven by molecular alterations creating clones with cellular plasticity, followed by additional events that lead to the treatment-emergent small-cell Neuroendocrine Prostate Cancer (tNEPC)11. Most NEPC cases are diagnosed in advanced stages with metastasis explaining the high mortality rate 11-15. While de novo NEPC are rare16, 17, tNEPC account for approximately 20% of advanced, treatment-refractory CRPC cases5, 7, 18 and this number may further rise as a result of hormone therapies moving earlier in the line of treatment19.
The Epigenetic Landscape of NEPC
The genomic variations between CRPC and NEPC are limited, except for the frequent loss of tumor suppressors PTEN, RB1, and TP53. However, the progression to NEPC is accompanied by extensive transcriptional reprogramming 11, 15, suggesting that the emergence of the neuroendocrine phenotype is driven predominantly by epigenetic dysregulation20-23. Epigenetic plasticity controls cell fate by altering DNA accessibility to transcription factors and gene expression. The process is controlled through regulation of DNA methylation, nucleosome remodeling, and histone modifications24. Altogether, alterations in the epigenome of CRPC tumors following AR-targeted therapies is characteristic of the progression to tNEPC.
Chromatin remodeling and the role of Transcription factors
The progression of CRPC to tNEPC under the pressure of ARPIs is marked by significant chromatin remodeling, where the chromatin accessibility increases at regions regulating the stem-cell and neuronal pathways, to facilitate the binding of specific transcription factors (TFs). This creates the shift in gene expression patterns towards a neuronal phenotype. During the progression to NEPC, ASCL1, a neuronal-determinant TF, is central to driving lineage plasticity and neuronal lineage determination. ASCL1 expression, motif accessibility, and activity increase disproportionately following ARPIs, leading to the activation of stem-cell-like and neuronal programs. Notably, loss of ASCL1 in NEPC results in the significant loss of accessible chromatin and reversal of the neuronal state to luminal one22. ASCL1 further support the NEPC phenotype by reprogramming the cistrome of other pioneering TFs such as FOXA125 . Therefore, the transition from CRPC to NEPC provoked by ARPIs modify the transcriptional control of lineage specific transcription factors.
It is important to highlight the distinction among NEPC subtypes through their chromatin landscape, since each subtype will have its unique vulnerabilities that can be explored for therapeutic purposes. Although converging towards a neuronal program, neuronal-lineage determinant TFs such as ASCL1 and NEUROD1 orchestrate divergent chromatin remodeling trajectories that favor the neuroendocrine (NE) program. Further, investigations employing single-cell chromatin accessibility analyses in mouse models have unveiled two distinct clones, driven by ASCL1 or the stem-cell specific TF OCT11 (also known as POU2F3), which defines a non-NE subtype. These results emphasize the complexity of the epigenetic landscape in NEPC but also highlights the specificity of TFs' involvement in driving the NE phenotype, highlighting the nuanced interplay of epigenetic mechanisms in cancer progression.
Epigenetic modifiers
Lineage determinant TFs guide specific chromatin remodelers and epigenetic modifiers to exert their influence, thereby establishing the desired cell fate. Epigenetic modifiers governing prostate cancer lineage switches are often dysregulated in NEPC, such as histone methyltransferase EZH2 and DNA methyltransferases26, 27. EZH2, a core component of the Polycomb Repressive Complex 2 (PRC2), deposits methyl groups on lysine 27 of histone 3 (H3K27) to suppress transcription28. EZH2 is over-expressed in NEPC compared to CRPC and plays a role in silencing the AR-driven luminal program. Interestingly, EZH2 has been reported to function in a non-canonical manner, in concert with an alternative AR program, to induce histone acetylation and facilitate the NE lineage conversion to activate stem-cell and NE programs 23.
In parallel, the role of DNA methylation in distinguishing prostate cancer stages has become increasingly evident11, 29. DNA methylome alterations is a hallmark of tNEPC, highlighting the unique epigenetic profile of these tumors11. In fact, a distinct DNA methylation pattern (combination of DNA hyper- and hypo-methylation) is associated with NEPC, which can be detected in circulating cell-free DNA (cfDNA), potentially serving as significant biomarkers29, 30. However, whether NEPC subtypes can be distinguished with DNA methylation patterns remain to be elucidated. These intricate interactions between lineage-determining TFs and epigenetic modifiers underscore the complexity of prostate cancer progression and the development of lineage plasticity. Given the potential reversibility of epigenetic modifications, unraveling mechanisms controlling lineage plasticity could lead to the identification of therapeutic targets and the development of strategies to combat resistance to ARPIs.
Learn more:
These review articles summarise the transcriptional and epigenetic mechanisms governing prostate cancer lineage plasticity:
- The Transcriptional and Epigenetic Landscape of Cancer Cell Lineage Plasticity | Cancer Discovery | American Association for Cancer Research (aacrjournals.org)
- Epigenetics in prostate cancer: clinical implications - PMC (nih.gov)
- Epigenetic reprogramming during prostate cancer progression: A perspective from development - PMC (nih.gov)
Images created in www.BioRender.com
Acknowledgments
We would like to express our gratitude to Dr. Steve Bilodeau for his valuable insights and expert review, which significantly enhanced the quality of this work. A special thank you also goes to Dr. Mathieu Schulz for the French translation, making this piece more accessible to the Francophone community.
References:
- Scher, H.I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med367, 1187-1197 (2012).
- de Bono, J.S. et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 364, 1995-2005 (2011).
- Kaarijarvi, R., Kaljunen, H. & Ketola, K. Molecular and Functional Links between Neurodevelopmental Processes and Treatment-Induced Neuroendocrine Plasticity in Prostate Cancer Progression. Cancers (Basel)13 (2021).
- Vlachostergios, P.J., Puca, L. & Beltran, H. Emerging Variants of Castration-Resistant Prostate Cancer. Curr Oncol Rep 19, 32 (2017).
- Aggarwal, R. et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-institutional Prospective Study. J Clin Oncol 36, 2492-2503 (2018).
- Beltran, H. et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 1, 487-495 (2011).
- Aparicio, A., Logothetis, C.J. & Maity, S.N. Understanding the lethal variant of prostate cancer: power of examining extremes. Cancer Discov 1, 466-468 (2011).
- Zou, M. et al. Transdifferentiation as a Mechanism of Treatment Resistance in a Mouse Model of Castration-Resistant Prostate Cancer. Cancer Discov 7, 736-749 (2017).
- Ellis, L. & Loda, M. Advanced neuroendocrine prostate tumors regress to stemness. Proc Natl Acad Sci U S A112, 14406-14407 (2015).
- Crona, D.J. & Whang, Y.E. Androgen Receptor-Dependent and -Independent Mechanisms Involved in Prostate Cancer Therapy Resistance. Cancers (Basel) 9 (2017).
- Beltran, H. et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med 22, 298-305 (2016).
- Abdulfatah, E. et al. De novo neuroendocrine transdifferentiation in primary prostate cancer-a phenotype associated with advanced clinico-pathologic features and aggressive outcome. Med Oncol 38, 26 (2021).
- Wang, H.T. et al. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J Clin Oncol 32, 3383-3390 (2014).
- Beltran, H. et al. Impact of Therapy on Genomics and Transcriptomics in High-Risk Prostate Cancer Treated with Neoadjuvant Docetaxel and Androgen Deprivation Therapy. Clin Cancer Res 23, 6802-6811 (2017).
- Labrecque, M.P. et al. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J Clin Invest 129, 4492-4505 (2019)
- Helpap, B., Kollermann, J. & Oehler, U. Neuroendocrine differentiation in prostatic carcinomas: histogenesis, biology, clinical relevance, and future therapeutical perspectives. Urol Int 62, 133-138 (1999).
- Zaffuto, E. et al. Contemporary Incidence and Cancer Control Outcomes of Primary Neuroendocrine Prostate Cancer: A SEER Database Analysis. Clin Genitourin Cancer 15, e793-e800 (2017).
- Epstein, J.I. et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol 38, 756-767 (2014)
- Linder, S. et al. Drug-Induced Epigenomic Plasticity Reprograms Circadian Rhythm Regulation to Drive Prostate Cancer toward Androgen Independence. Cancer Discov 12, 2074-2097 (2022).
- Davies, A., Zoubeidi, A. & Selth, L.A. The epigenetic and transcriptional landscape of neuroendocrine prostate cancer. Endocr Relat Cancer 27, R35-R50 (2020).
- Chakraborty, G., Gupta, K. & Kyprianou, N. Epigenetic mechanisms underlying subtype heterogeneity and tumor recurrence in prostate cancer. Nat Commun 14, 567 (2023).
- Nouruzi, S. et al. ASCL1 activates neuronal stem cell-like lineage programming through remodeling of the chromatin landscape in prostate cancer. Nat Commun 13, 2282 (2022).
- Davies, A. et al. An androgen receptor switch underlies lineage infidelity in treatment-resistant prostate cancer. Nat Cell Biol 23, 1023-1034 (2021).
- Bauer, A.J. & Martin, K.A. Coordinating Regulation of Gene Expression in Cardiovascular Disease: Interactions between Chromatin Modifiers and Transcription Factors. Front Cardiovasc Med 4, 19 (2017).
- Baca, S.C. et al. Reprogramming of the FOXA1 cistrome in treatment-emergent neuroendocrine prostate cancer. Nat Commun 12, 1979 (2021).
- Ku, S.Y. et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 355, 78-83 (2017)
- Smith, B.A. et al. A Human Adult Stem Cell Signature Marks Aggressive Variants across Epithelial Cancers. Cell Rep 24, 3353-3366 e3355 (2018).
- Akamatsu, S., Inoue, T., Ogawa, O. & Gleave, M.E. Clinical and molecular features of treatment-related neuroendocrine prostate cancer. Int J Urol 25, 345-351 (2018).
- Beltran, H. et al. Circulating tumor DNA profile recognizes transformation to castration-resistant neuroendocrine prostate cancer. J Clin Invest 130, 1653-1668 (2020).
- Berchuck, J.E. et al. Detecting Neuroendocrine Prostate Cancer Through Tissue-Informed Cell-Free DNA Methylation Analysis. Clin Cancer Res 28, 928-938 (2022).